About
Bio
FDAnews.com covers domestic and international regulatory, legislative and business news and information for executives in industries regulated by the U.S. Food and Drug Administration, including four newsletters: Drug GMP Report, Drug Industry Daily, International Devices & Diagnostics Monitor and The GMP Letter.
Email
email@cision.one
Website
site@cision.one
Social media




Location
United States of America
Frequency
upgrade
Circulation
upgrade
Sectors
Government Agencies, Medical Equipment, Medical Technology, Pharmaceutical Manufacturers
Bio
FDAnews.com covers domestic and international regulatory, legislative and business news and information for executives in industries regulated by the U.S. Food and Drug Administration, including four newsletters: Drug GMP Report, Drug Industry Daily, International Devices & Diagnostics Monitor and The GMP Letter.
Website
Social media
Location
Frequency
Circulation
Sectors
Government Agencies, Medical Equipment, Medical Technology, Pharmaceutical Manufacturers
Most recent articles by International Devices & Diagnostics Monitor
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Article description
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Article description
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Article description
Explore outlets similar to International Devices & Diagnostics Monitor
-
 National Review
National ReviewDesigned as a journal of conservative opinion. Covers the national and international developments related to conservative action and reports and analyzes on what various conservatives are saying, thinking and doing. Committed to providing important, expert analyses of the day’s hottest stories and to breaking those stories in a manner consistent with the publication's worldview.
ViewPPopular MechanicsPublished by Hearst and established in 1902, Popular Mechanics is designed to satisfy the reader's passion to know how and why things work. The publication covers how today's technologies affect the things readers are most interested in: their homes, consumer electronics, cars, science, computers, sports, and current events, and explains the way the world works in the 21st century. Editorial engages readers with breakthroughs on the latest advances in science and technology, and educates with informative how-to stories on digital technology, automotive, and the home. It also motivates minds to act with product reviews and comparisons on all of the latest equipment and products. Articles are broken into five departments: - Technology: covering the Internet, personal computers, computer games, and other consumer electronics - Automotive: featuring articles about new car models, motor sports, and automotive repair and maintenance - Science: covering general interest health, technology, and physical science topics - Home: offering home maintenance and renovation information - Outdoors: covering sports and outdoor recreation Other editorial sections include: - Technology Watch: a front-of-book section offering short news items on technology, aviation, environment, energy, computers, robotics, space, transportation, health, and military - Spy Report: comprehensive sneak peeks of cars and trucks coming out of the U.S., Europe, and Asia - Great Stuff: listing new home, automotive, recreation, consumer electronics, and fitness products - Time Machine: revisiting article topics from 90, 60, and 30 years ago
View Industry Dive
Industry DiveIndustry Dive is a Business news publication that takes a deep dive into 16 of the most competitive industries. Industries included on website: BioPharma, CIO, Construction, Education, Food, Grocery, HR, Healthcare, Marketing, MedTech, Restaurant, Retail, Smart Cities, Supply Chain, Utility, and Waste.
View Thomson Reuters Foundation
Thomson Reuters FoundationBackground and Format The Thomson Reuters Foundation, the charitable arm of Thomson Reuters, covers the world’s under-reported stories, with a global team of about 40 staff journalists on humanitarian crises, women’s empowerment, the human impact of climate change, property rights, and trafficking and slavery. All of its stories are published on its news website, news.trust.org, as well as distributed on the Reuters news wire. Established in 1983 as a training programme for journalists in developing countries, the Thomson Reuters Foundation promotes free, independent journalism through its continued training programmes and also acts to promote socio-economic progress worldwide. As well as its news service and journalism training, the Foundation runs Trust Law - which connects law firms providing pro bono work to NGOs and social enterprises - and holds the annual Trust Women conference focused on empowering women and combating trafficking and slavery. Trust.org is the Foundation’s main website. Audience and Readership: People interested in humanitarian stories as well as campaigners and lawyers along with people working in law enforcement, politics and global business. Deadline: Best to contact in the morning. Frequency: Daily - online. Monthly Unique Users: This figure is not available. Distribution: Content is produced for the website and also distributed through the Reuters news wire. Awards: 2013 - Online Media Awards, Best National/World News Site: for Aswat Masriya (nominated) Other Information: The Foundation hold an annual conference called Trust Women which brings together campaigners, filmmakers, lawyers, senior figures from law enforcement, politics, the judiciary and global business to take action to advance the status of women and their rights. PRs can get involved by sending us ideas for speakers - individuals who are leaders in their field – to media.foundation@thomsonreuters.com. It also provides the TrustLaw global pro bono legal programme connecting NGOs and social entrepreneurs with big law firms. They like to receive new ideas from PRs and quirky stories. Embargoed content is preferable - or to at least receive a story well ahead of time. They're particularly interested in 'people stories', a story that has a human face. Whatever they receive, it must fit into their remit, content has to have a global view and focus on under reported stories, humanitarian relief, governance, trafficking and slavery, and must promote socio-economic progress. The team are also available to moderate at presentations and conferences if it can promote the Foundation.
View Modern Healthcare
Modern HealthcareEstablished in 1974 and written for hospital and health system executives as an industry-intensive news source. Editorial concentrates on issues, news and trends affecting healthcare in the United States and provides information to healthcare professionals on topics including finance, integrated delivery systems, technology, politics, labor, managed care, physician issues, developments in federal and state government, court rulings, policy and regulation and marketing. There is a particular emphasis on coverage of electronic medical records. Regular features provide up to the minute news and trends in the healthcare field and discusses physician issues, innovative strategies and studies, future developments within this field and personnel updates. Departments include: The Week in Healthcare, Late News, Opinions/Editorials, Cover Story and Features. Topics regularly covered include: human resources, finance, legal affairs, managed care, policy and technology. Special sections included are: Profile, an in-depth look at a healthcare industry leader; Medical Advances, how the latest developments in medicine, biotechnology, pharmaceuticals and medical technology will affect your business; By the Numbers, a look at healthcare statistics and figures; and The Edge, how information technology is helping healthcare management solve clinical, financial and strategic challenges. Modern Healthcare does not publish unsolicited news articles or "byline" articles from outside firms. All of the editorial content is developed by staff or commissioned from freelance writers.
ViewUse CisionOne to find more relevant outletsExplore journalists that write for International Devices & Diagnostics Monitor
Contact us to find more relevant journalistsContact International Devices & Diagnostics Monitor and get access to over 850K accurate, up-to-date media profiles.
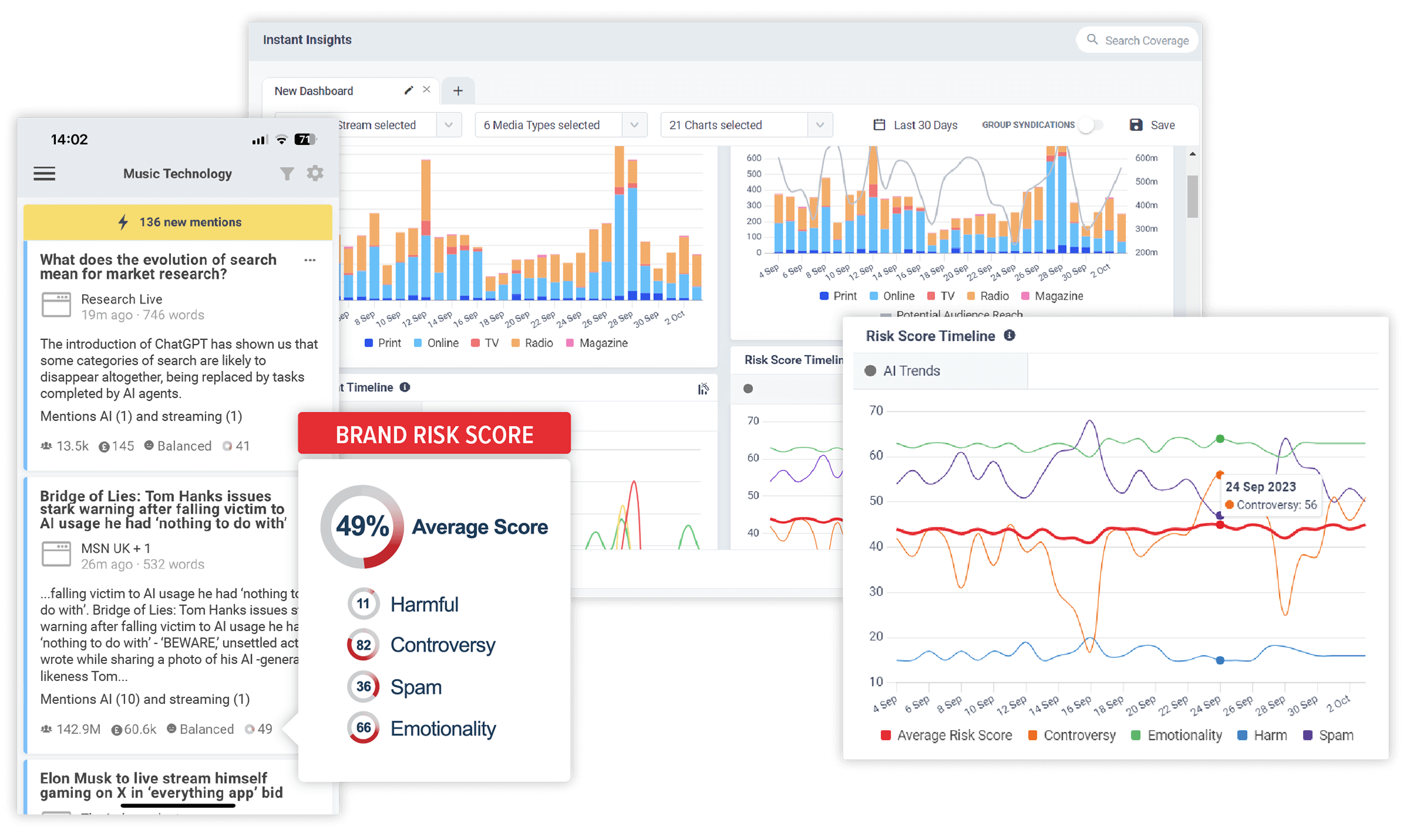
Discover the stories that impact your brand. In realtime.
CisionOne delivers relevant news, trends and conversations that matter to your brand with the world’s most comprehensive media monitoring service across Print, Online, TV, Radio, Social, Magazines, Podcasts and more.